
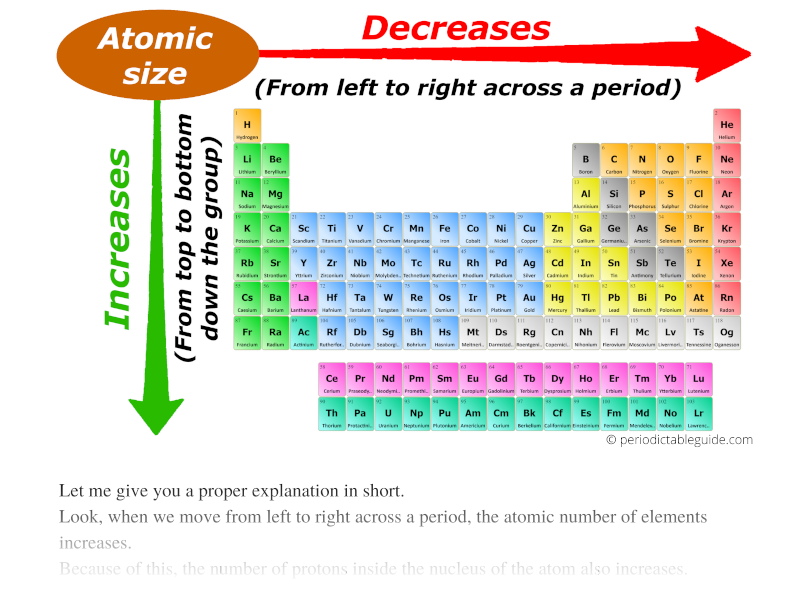
We will discuss the following periodic properties in the modern periodic table. Elements are placed in groups and periods on the basis of similar properties and systematic variation of properties.
In the Modern Periodic Table, the elements are arranged in the ascending order of their atomic number. It is of worth importance to know about The variation of different physical and chemical properties in the modern periodic table including groups and periods is known as the periodicity of properties. Periodic trends in the modern periodic table are the basics of chemistry. Trend of melting and boiling points in group.
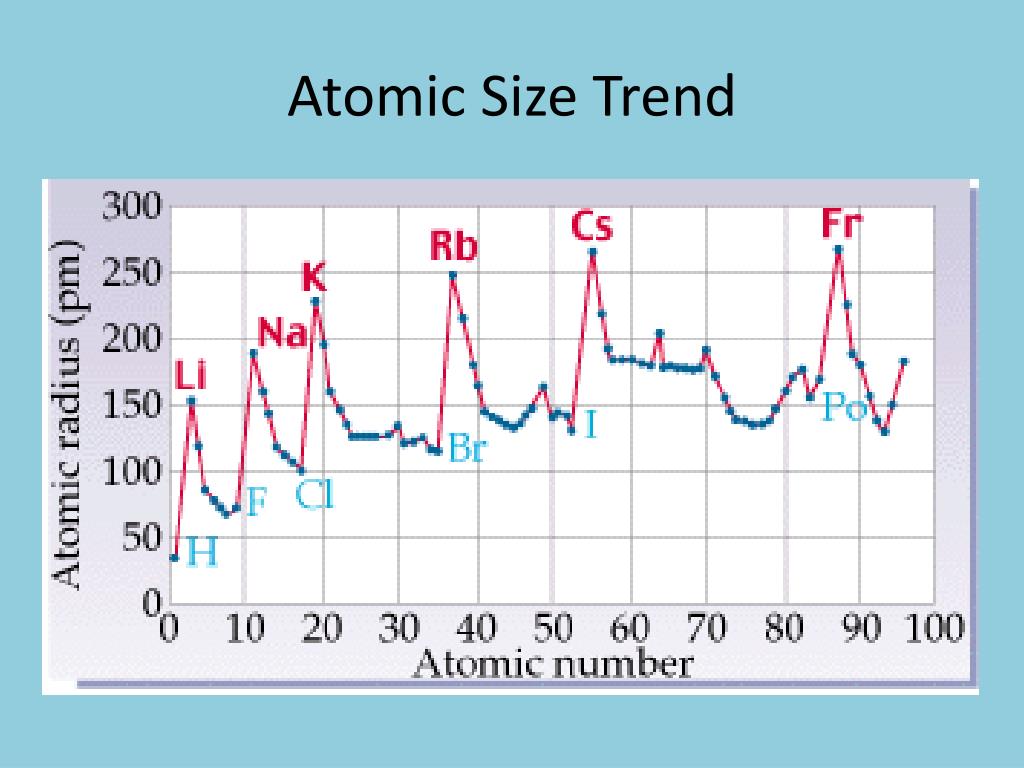
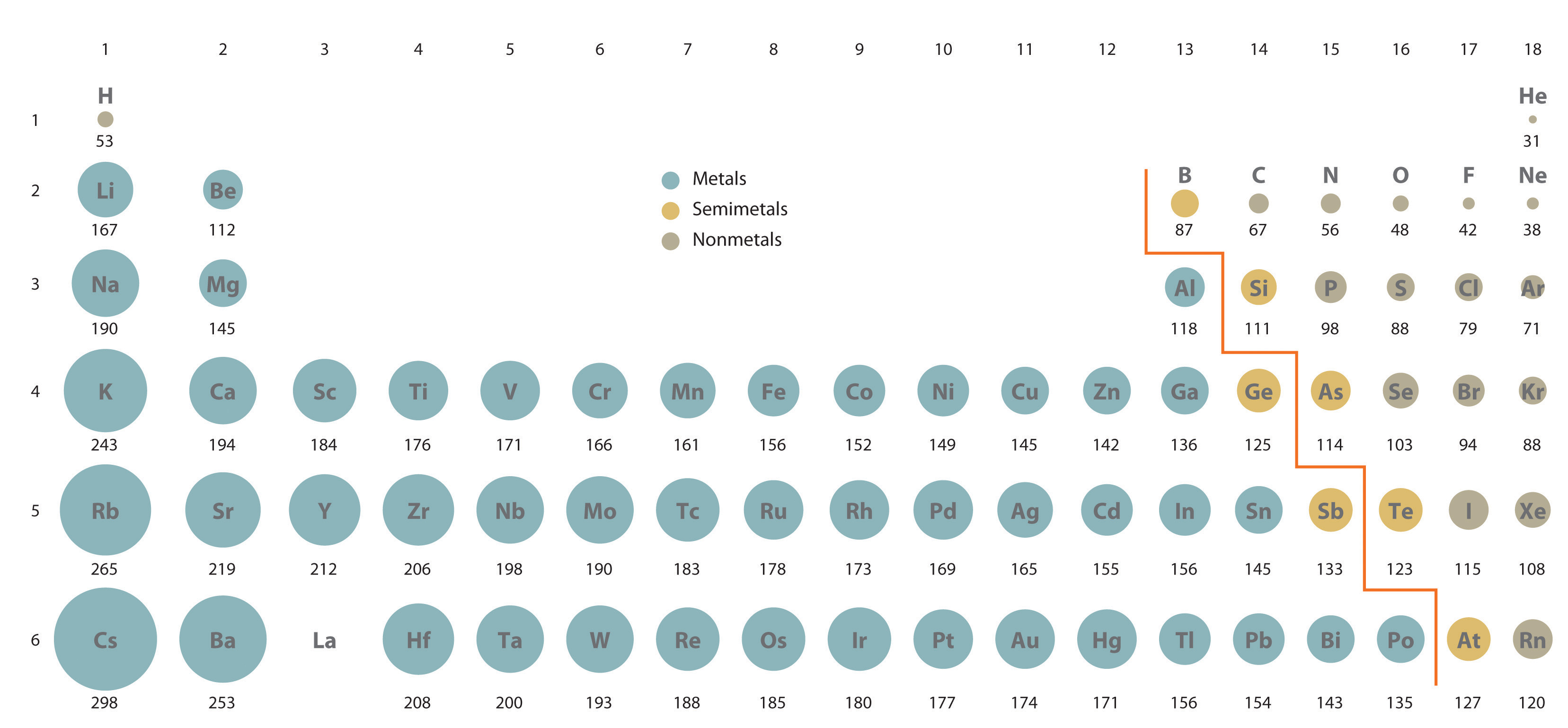
Variation of melting and boiling point in a group.Graphical explanation melting and boiling point in periods.Trend of melting and boiling point in periods.Periodic Trends of melting and boiling point.Non-metallic character trend in periodic table.Metallic character trend in the periodic table.Metallic character trend in periodic table.Factors affecting the electron affinity.Electron affinity trend in periodic table.Graphical explanation of ionization energy.Variation of ionization energy values in the periodic table.Factors affecting the ionization energy values.Ionization energy trend in the periodic table.Graphical representation of atomic radii.Comparative study of ionic radii vs atomic radii.Ionic radius trends in the periodic table.Atomic radius trends in transition elements.Why atomic radius decreases in a period?.Atomic radius trends in the periodic table.


 0 kommentar(er)
0 kommentar(er)
